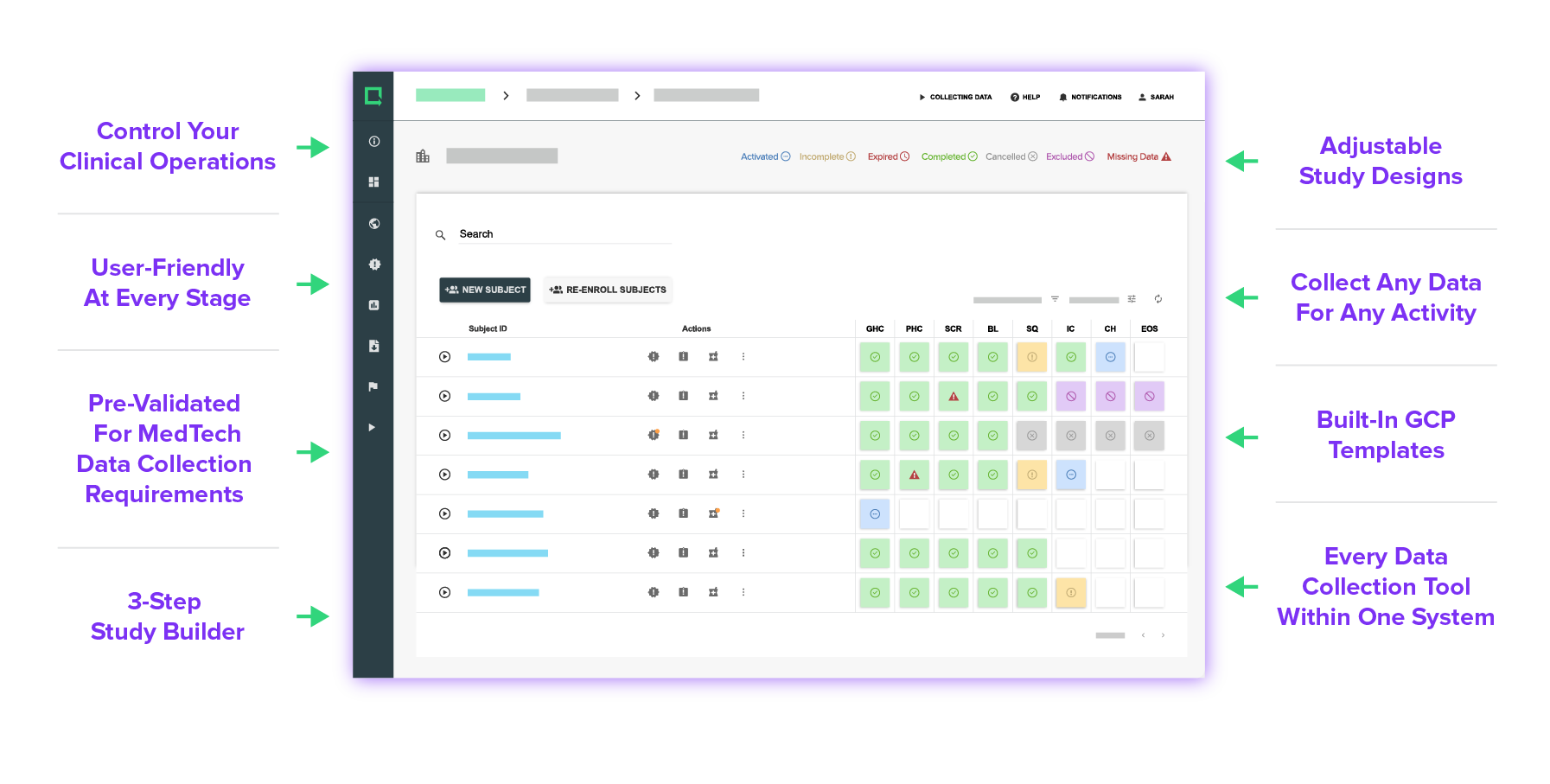
Every Feature Made for MedTech
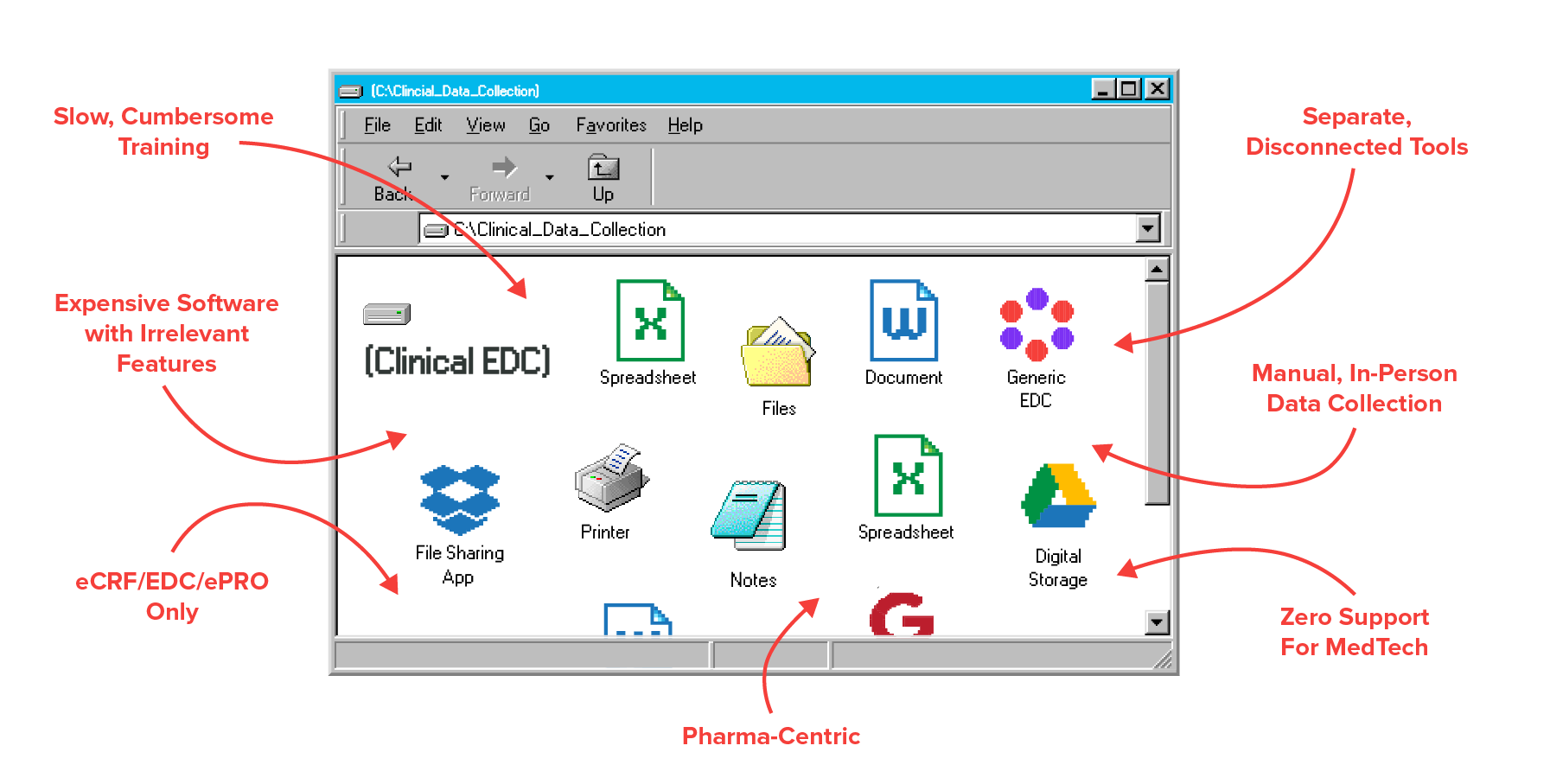
Your software is already optimized for medical device studies. That means no coding, no stressful setup, and no expensive pharma-centric features you'll never use.
Ensure compliance from the start with built-in ISO 14155:2020, EU MDR, and FDA requirements. In addition, you'll get 13 ready-to-use and customizable compliance document templates.
Easily make adjustments to your study designs according to different countries' requirements and EC approvals.
If your study subjects require one or many treatments with your device, you can use multiple activations for any visit event.
Set up a study in as little as 90 seconds with intuitive drag-and-drop elements, form validation, and customizable protocol designs. Adjust and reuse your studies as much as you need to.
Functionality Meets Convenience
Experience versatile tools and customizations that anyone can use and build your study exactly as you need it.
Design your eCRF based on your unique study requirements — simple or complex, there are no limits.
Learn MoreCreate a user-friendly experience for study subjects and increase response rates, adherence, and quality of data.
Out-of-the-box GCP-compliant surveys. Collect data with dynamic surveys to comply with EU MDR PMCF or FDA Post-Approval requirements.
Learn MoreThe mobile-first case studies series data collection tool you've always wanted. Clinician-friendly and compliant. Enable ad-hoc data entry of high-quality patient data in post-market settings.
Learn MoreFrom eConsent to randomization, custom study dashboards, and external system integrations.
Learn MoreHelping MedTech Companies
Make an Impact
See how some of the world's most advanced medical device companies use
Greenlight Guru Clinical.